Tunnel-type Sodium Iron Phosphates as Electrodes in Sodium Iron Batteries using Pyrrolidinium-based Ionic Liquid Electrolyte
Trajche Tushev, Sonya Harizanova, Violeta Koleva, Radostina Stoyanova
Abstract: Besides the positive and negative electrodes, the electrolyte is a key component in lithium/sodium ion batteries (LIB/SIB) as it provides the ion transport between the two electrodes in the electrochemical cell. Recently, ionic liquids (ILs) based on imidazolium and pyrrolidinium cations have been proposed as promising alternative to the conventional organic electrolytes (often NaPF6 or NaClO4 salts dissolved in a mixture of organic carbonates such as propylene carbonate, ethylene carbonate, diethyl carbonate, etc.). ILs are non-flammable, non-volatile, with excellent thermal and electrochemical stability, enabling battery operation at higher temperatures (40-90°C) and within wide electrochemical windows (up to 6V). These properties make ILs safety and green alternative to the organic electrolytes, but the high manufacturing cost currently limits their large-scale application.
To date, only limited phosphate-based positive electrode materials such as NaFePO4, Na2FeP2O7 and Na3V2(PO4)3 have been investigated using ILs as electrolytes and very promising electrochemical characteristics in terms of energy density and capacity retention that outperform the organic electrolyte-based devices have been reported.
Here, we first report electrochemical properties of tunnel–type sodium iron phosphates, Na4Fe3(PO4)2P2O7 and Na2Fe3(PO4)3 (Fig. 1), in sodium half-cells using NaFSI:[Pyr13]+[FSI]-electrolyte (Pyr13FSI is N-methyl-N-propylpyrrolidinium bis-(fluorosulfonyl)imide) (1:9 mole ratio).
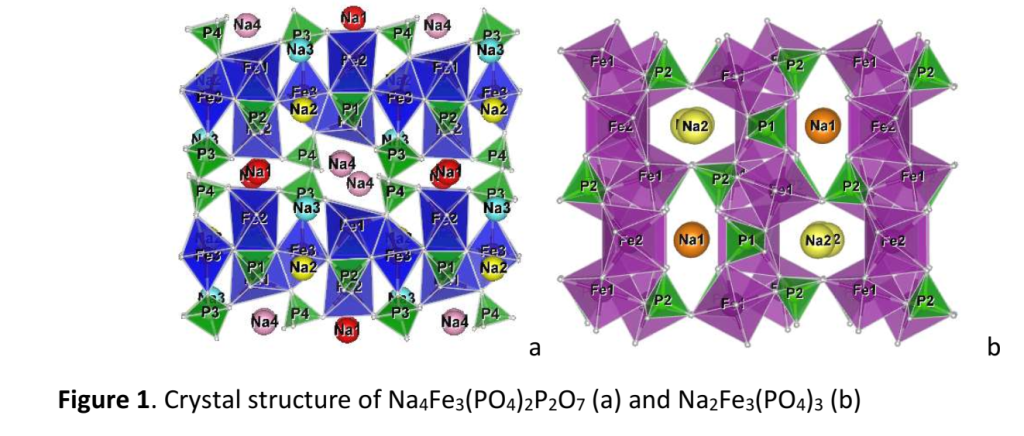
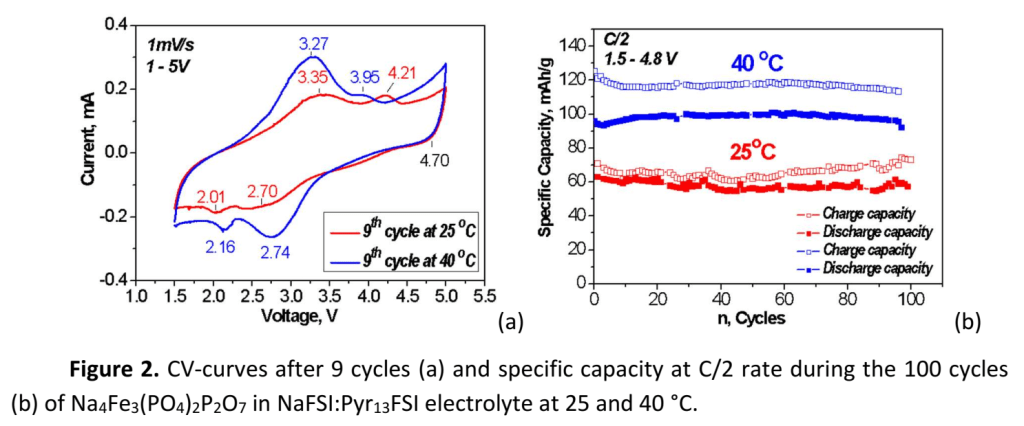
The phosphate materials are prepared by thermal decomposition and further annealing at 500-600 °C of the phosphate-formate precursors, the latter being obtained by freeze-drying of solutions containing NaH2PO4, Fe(HCOO)2.2H2O and (NH4)2HPO4 in needed proportions. The electrochemical performance is studied in galvanostatic and potentiostatic regimes at 25 and 40 °C in different voltage windows. The observation of clear redox peaks in the CV curves owing to Fe2+/Fe3+ redox pairs, that remain unchanged during the cycling, confirms the electrochemical activity and stability of the phosphates in the NaFSI:Pyr13FSI electrolyte (Fig. 2a). An improved charge-discharge performance is established at elevated temperature: the reversible capacity is about 100 mAh/g at C/2 rate after 100 cycles (Fig. 2b).
The phase stability and surface species formed during the cycling in IL electrolyte have been studied by ex-situ XRD and XPS techniques. The results obtained demonstrate the potential of tunnel-type Na4Fe3(PO4)2P2O7 and Na2Fe3(PO4)3 as cathodes in SIBs using NaFSI:Pyr13FSI electrolyte at intermediate temperature.
Acknowledgements: The authors thank to the financial support of project Д01-92/06.2022 (European Network on Materials for Clean Technologies, TwinTeam) as well as project CARiM (NSP Vihren, КП-06-ДB-6/16.12.2019) for the freeze-drying apparatus and project “Energy Storage and Hydrogen Energy” (NSIESHER, № Д01-161/28.07.2022) for incubators used.
The authors from CARiM’s Research Team are bolded.
