Electrode Potential Calculation Accounting for the Dielectric Medium of the Bulk Electrode
Ilia Kichev, Lyuben Borislavov, Radostina Stoyanova and Alia Tadjer
Abstract: Redox-active organic molecules are the major candidates for electrode material components in metal-ion organic batteries. Therefore, the importance of accurate theoretical assessment of their electrode potential utilising cheap and efficient methods is essential. Traditionally, the theoretical calculations of this quantity employ an implicit environment of the redox-active molecule, simulated as an isotropic electric field, which is produced by a medium with a predefined dielectric constant. The latter is usually equal to that of the electrolyte, motivating the choice with the argument that the reduction occurs at the electrode-electrolyte interface. However, the bulk electrode is also involved in the reduction and it may have vastly different properties compared to those of the electrolyte. Moreover, the dielectric characteristic of the bulk electrode certainly does not remain constant during the charge/discharge process.
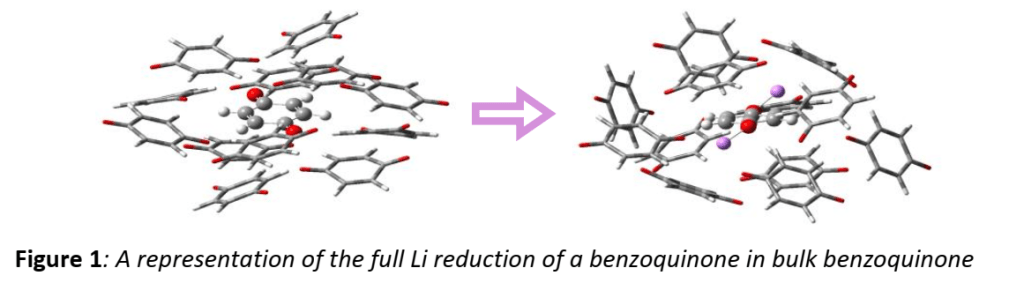
This study is focused on the development of a novel protocol for calculation of the electrode potential in the bulk of the electrode. On the one hand, standard DFT/PCM calculations were carried out searching for relationship between the electrode potential, the dielectric constant of the medium and the electric properties of the redox-active organic molecule before and after reduction with Li. On the other hand, a cluster cut out of the crystal structure of the target compound is modelled by means of an ab initio calculation with twolayered ONIOM (Figure 1) approach before and after the reduction of a separate molecule in it, in order to analyse the structural changes invoked by the reduction and their effect on the overall electrostatics.
Acknowledgements: The study is supported by the Bulgarian National Science Fund, Project CARiM/VIHREN, grant KP-06-DV-6/2019 and by Project D01-272/02.10.2020
The authors from CARiM’s Research Team are bolded.
