Carbon-Based Composites with Sodium Iron Phosphates with Improved Electrochemical Performance
Violeta Koleva, Sonya Harizanova, Trajche Tushev and Radostina Stoyanova
Abstract: The increasing demand for large-scale, low-cost, and efficient energy storage systems stimulate scientific and applied research on sodium transition-metal polyanion materials for battery application. The main representatives of polyanion-type electrodes are phosphate-based compounds. The interest in the phosphate electrodes is determined by their numerous advantages such as high structural and thermal stability of the phosphate matrix, safety, suitable work voltages, operation at higher temperatures, wide distribution of sodium in nature and low cost, particularly in the case of Fe-containing phosphates. To date, many studies have been conducted on iron-containing phosphate electrode materials, and some progress has been made. However, the typical low conductivity which leads to relatively low capacity performance of such materials still restrict their practical application.
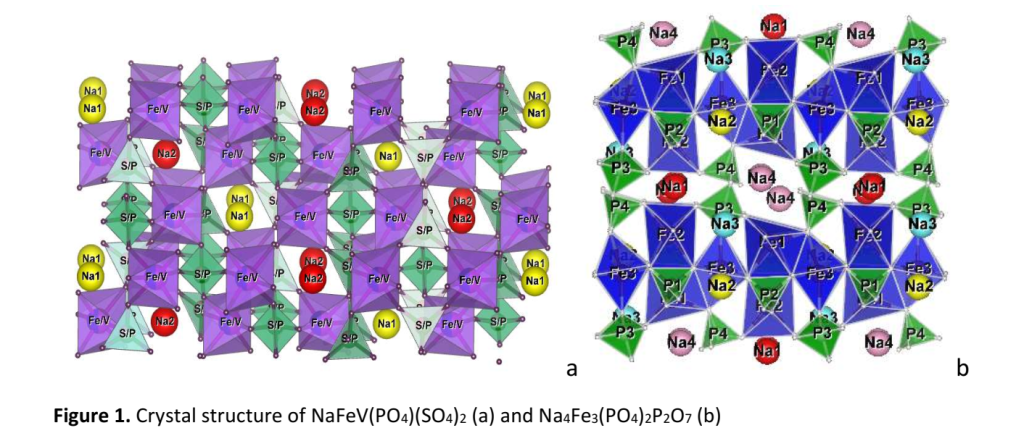
From a structural point of view, the phosphates having a tunnel-type structure that provides channels for fast mobility of alkali metal ions are among the most promising electrode materials for future battery application. In this regard, our attention is directed to two compounds which compositions contain two kinds of anionic units: NaFeV(PO4)(SO4)2 (labeled NFVPS) and Na4Fe3(PO4)2P2O7 (labeled NFPP). The tunnel-type structure of the two compounds can be clearly seen from the crystal structure representation (Fig. 1).
The two compounds are prepared by precursor method where the precursors are obtained by freeze-drying of solutions containing: NaH2PO4 and Fe(HCOO)2 (4:3 mole ratio) for NFPP and NaH2PO4, Fe(NO3)3, (NH4)2SO4 and NH4VO3 (1:1:2:1 mole ratio) for NFVPS, followed by annealing at 500 and 400 °C, respectively.
In order to increase the low electronic conductivities of NFPP and NFVPS two types of carbon-based composites have been prepared as cathode materials: i) with 15 % rGO (reduced graphene oxide), labeled as NFPP/G and NFVPS/G; ii) with 15 % carbon black labeled as NFPP/C and NFVPS/C. The composites are obtained via ball-milling procedure. Their structure and morphology have been studied by XRD and SEM. The electrochemical properties of the carbon composites have been examined in Li-half cells using LiPF6 in EC:DMC as electrolyte in galvanostatic and potentiostatic regimes at 25 and 40 C. The charge-discharge performance and cycling stability have been tested at C/2 rate (Figure 2).

The results reveal that rGO is more effective additive than carbon black in the achievement of higher specific capacity for both compounds. After 100 cycles the obtained discharge capacities are: 102 mAh/g for NFPP/G and 82 mAh/g for NFVPS/G. The composites with carbon black exhibit lower specific capacities: about 60 mAh/g for NFVPS/C and about 30 mAh/g for NFPP/C, but here the reversibility is better than in the case of composites based on rGO. The phase stability of the phosphates during the cycling has been followed by ex-situ XRD method.
In conclusion: The present study demonstrates the effectiveness of carbon-based composites with sodium iron phosphates for achievement of good electrochemical performance in hybrid metal-ion batteries.
Acknowledgements: The authors thank to the financial support of project CARiM (NSP Vihren, КП-06-ДB- 6/16.12.2019) for the freeze-drying apparatus used. The authors are also grateful to the project Д01- 92/06.2022 – “European Network on Materials for Clean Technologies”, to present the obtained results in the SizeMat4.
The authors from CARiM’s Research Team are bolded.
