Effect of the CeO2 coating on electrochemical performance of layered oxide cathode with composition Na2/3Mg1/3Mn2/3O2
Rositsa Kukeva, Mariya Kalapsazova and Radostina Stoyanova
Abstract: The layered materials provide a combination of high capacity, high voltage, and high stability and are widely studied in attempts to develop new cathode materials for sodium-ion batteries. The variety of existing layers’ arrangements opens potential for diversity in their properties, including their performance as an energy storage material. One of the candidates for a cathode material that crystallizes in P2 and P3 phases is a layered oxide, Na2/3Mg1/3Mn2/3O2, characterized by charge-discharge capacity provided by Mn4+/Mn3+ redox couple in a voltage range below 4.0 V and by O2-/O- redox couple at a voltage above 4.2 V.
The achievement of enhanced electrochemical performance by extending the upper voltage boundary leads to deterioration of the cathode surface layer, due to interfacial or surface reactions. The evolution of the interface depends on the active material composition and the nature of the electrolyte. Applying a cathode coating could reduce surface degradation of cathode and ensure a smoother surface. A variety of coating materials were studied, such as Al2O3, ZrO2, ZnO, CeO2, etc.
In this study, CeO2 was coated on the surface of Na2/3Mg1/3Mn2/3O2 particles (existing as P2 and P3 phase) via ethanol-assisted homogenization with Ce(CH3COO)3, until the ethanol evaporated. The dry precipitate is heated respectively at 600C (for the P3 phase) and 800C (for the P2 phase) and is used for cathode active mass preparation. The selection of CeO2 as a coating material is due to its high oxidation capacity, which originates from the rapid shift of the oxidation state between Ce3+ and Ce4+. Resorting to CeO2 coating in layered oxide materials aims to improve oxygen storage and stabilize layered structure.
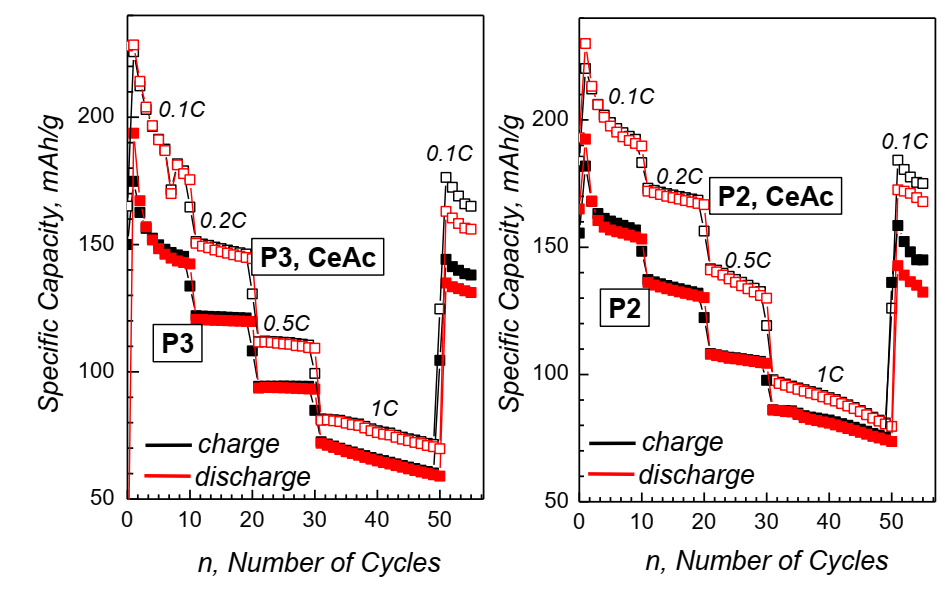
Fig.1. Cycling performance of pristine P3- and P2- phase (Na2/3Mg1/3Mn2/3O2) and ofP3 and P2 phase coated with CeO2
The surface-modified cathode materials show distinctively better electrochemical performance than
corresponding pure phases. The effect of CeO2 coating was further studied by ex-situ XRD, EPR, and SEM analysis of cathode material.
Acknowledgements: The financial support from Bulgarian National Science Fund, contract CARiM (NSP Vihren, ΚΠ-06-ДB-6/2019).
The authors from CARiM’s Research Team are bolded.
