How to stabilize oxygen redox activity in oxide electrodes?
Ekaterina Zhecheva, Rositsa Kukeva, Mariya Kalapsazova, Martin Nedyalkov, Radostina Stoyanova
Abstract: To double the specific capacity of intercalation-type electrodes (i.e. layered Li1+xTM1-xO2 and NaxTMO2) there is a need to activate simultaneously of cationic and anionic redox reactions during the alkali ion intercalation. The main drawbacks of layered oxides operating with simultaneous TM and oxygen redox reactions are their poor reversibility and cycling stability. This is related with oxygen redox activity that, on its turn, enforces inevitably an enhancement in the surface reactivity of the oxides due to the creation of hole and/or peroxide species. The occurrence of over-oxidized oxygen destabilizes the layered structure, culminating in oxygen gaseous release at potential higher than 4.5 V versus Li/Li+. In addition, the over-oxidized oxygen interacts easily with organic-based electrolytes leading to worsening of the cycling performance. Therefore, the enhanced surface reactivity of oxides will devaluated the unique properties of Li1+xTM1-xO2 and NaxTMO2.
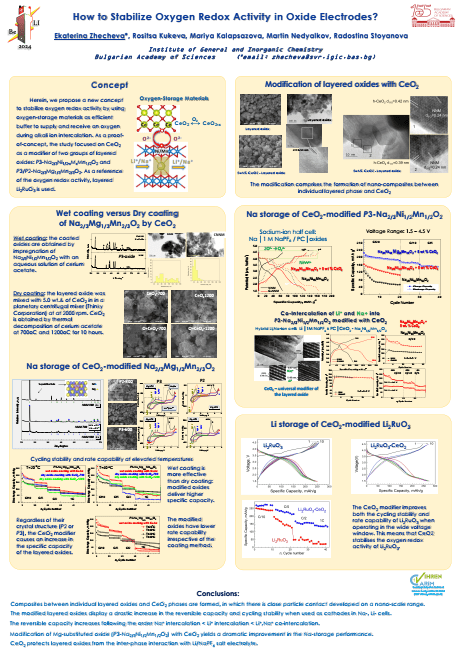
Herein, we propose a new concept to stabilize oxygen redox activity by using oxygenstorage materials as efficient buffer supplying and receiving an oxygen during alkali ion intercalation. As a proof-of-concept, the study is focused on CeO2 as a modifier of two groups of layered oxides: P3-Na2/3Ni1/2-xMxMn1/2O2, P3/P2-Na2/3Mg1/3Mn2/3O2 and Li2RuO3 (used as reference for oxygen redox activity). The surface modification relies on the impregnation of layered oxides with cerium acetate, followed by thermal treatment at 700 oC [1-2]. This method yields composites between individual layered oxide and CeO2 phases. In the composites, there is a close particles contact, which is developed on a nano-scale range.
The modified oxides display drastic increase in the reversible capacity and improved cycling stability when used as cathodes in Na-, Li- and hybrid Li,Na-ion cells. The large and stable reversible capacity is a result from the redox reactions with participation of nickel, manganese and ruthenium ions (between 1.5 and 4.2V) and lattice oxygen (above 4.2 V), the latter being stabilized by CeO2 modifier. The highest capacity of about 285 mAh/g is reached for CeO2-Na2/3Ni1/2Mn1/2O2 cycled in hybrid Li,Na-ion cell. The reversible capacity increases following the order Na+ intercalation < Li+ intercalation < Li+,Na+ co-intercalation. This is an experimental evidence supporting the concept on the positive impact of oxygen-storage oxides such as CeO2 on the oxygen-redox reaction in layered oxides. In addition, CeO2 protects NNM from the inter-phase interaction with Li/NaPF6 salt electrolyte. These data could serve as guidelines to achieve colossal reversible capacity at layered lithium and sodium transition metal oxides – research dream from the beginning until now in the development of lithium-ion batteries.
References
[1] M. Kalapsazova, K. Kostov, R. Kukeva, E. Zhecheva, R. Stoyanova, Oxygen-storage materials to stabilize the oxygen redox activity of three-layered sodium transition metal oxides. J. Phys. Chem. Lett. 12 (2021) 7804.
[2] M. Kalapsazova, R. Kukeva, E. Zhecheva, R. Stoyanova, Metal Substitution versus OxygenStorage Modifier to Regulate the Oxygen Redox Reactions in Sodium-Deficient Three-Layered Oxides, Batteries 8 (2022) 56.
ACKNOWLEDGMENT. The authors acknowledge the financial support of the Bulgarian National Science Fund, contract CARiM (NSP Vihren, КП-06-ДВ-6).
The authors from CARiM’s Research Team are bolded.